MEDIFUGE & CGF KIT Patent pending - Blood Phase Separator
|
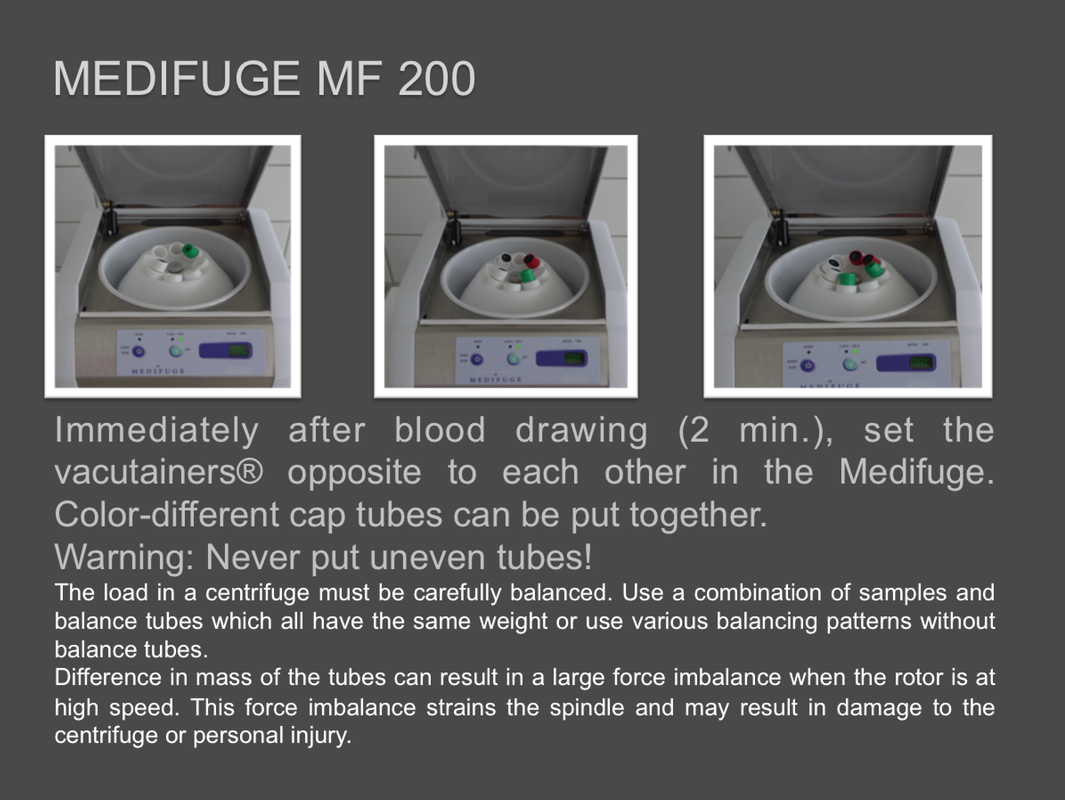
Rotor Phase separator:
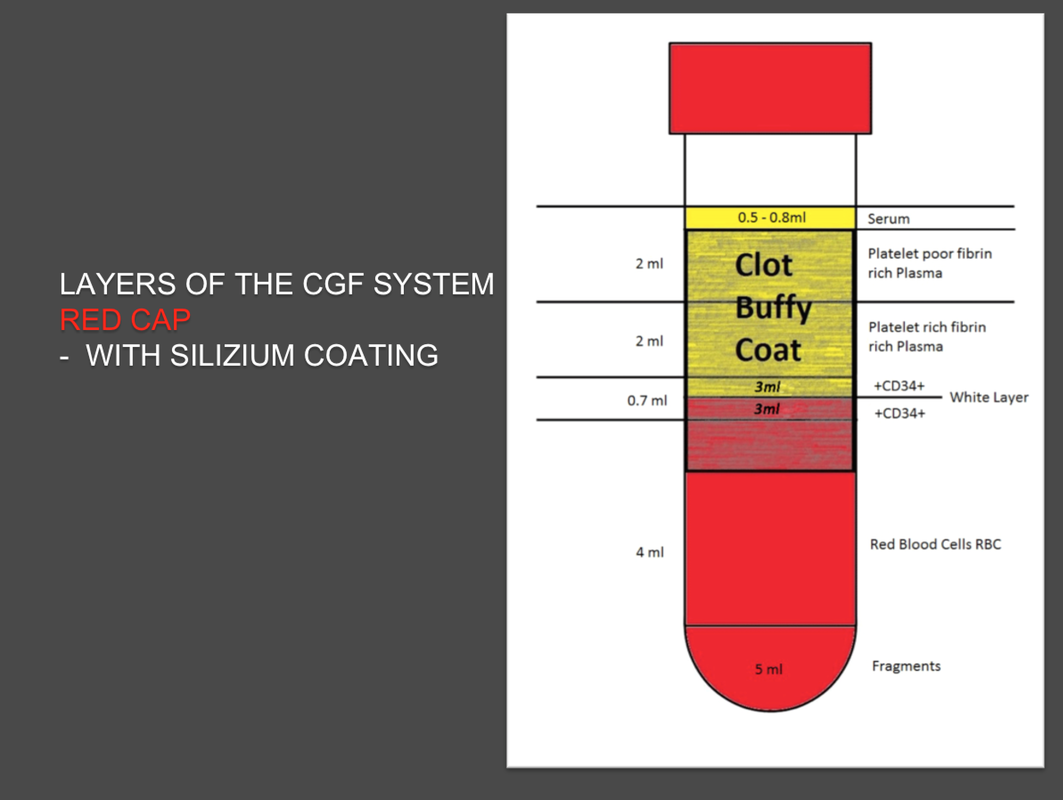
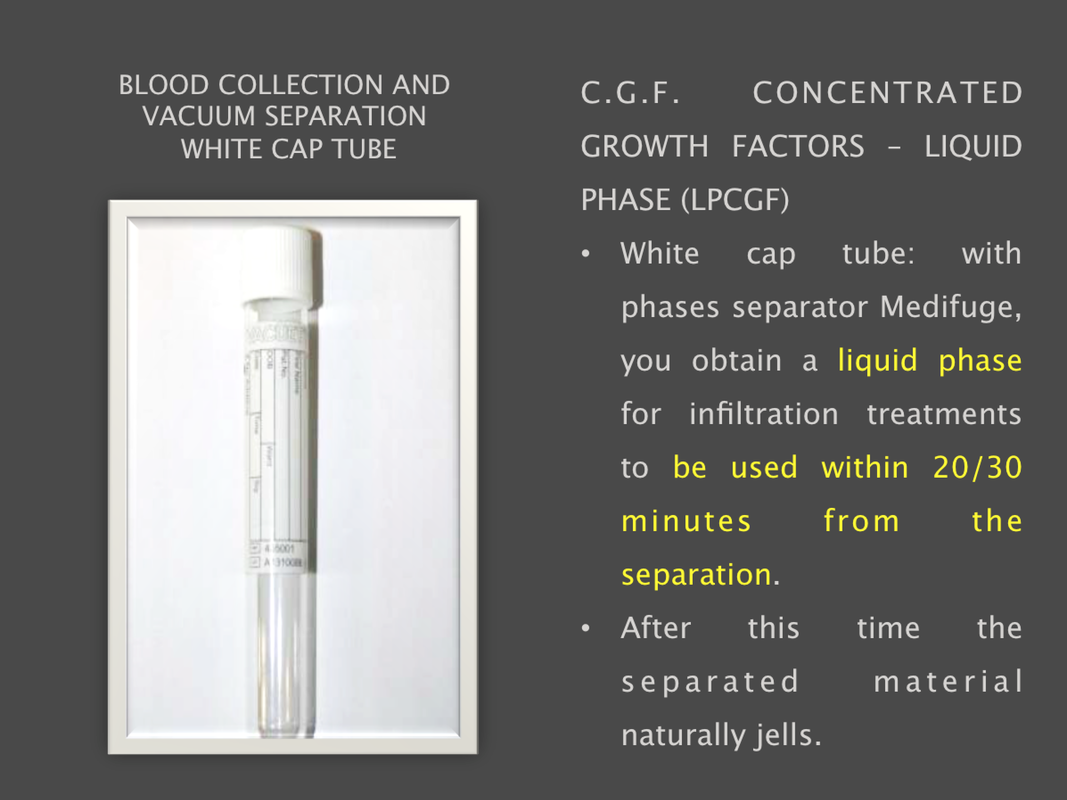
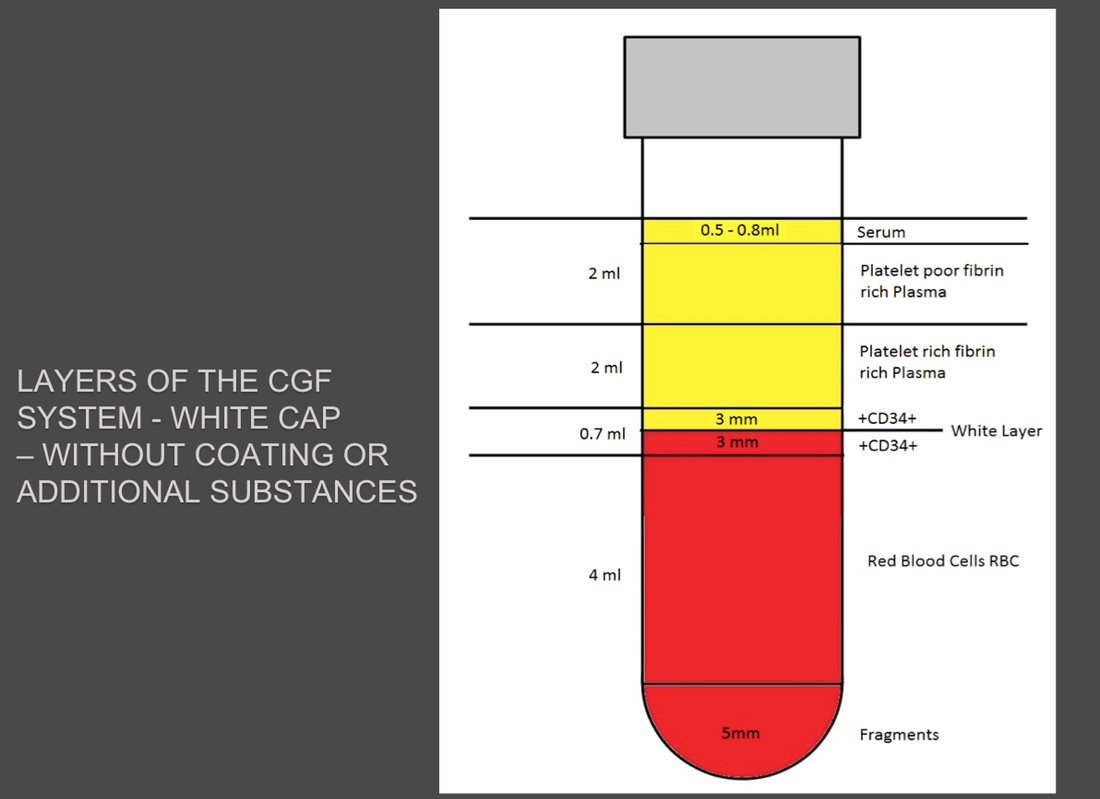
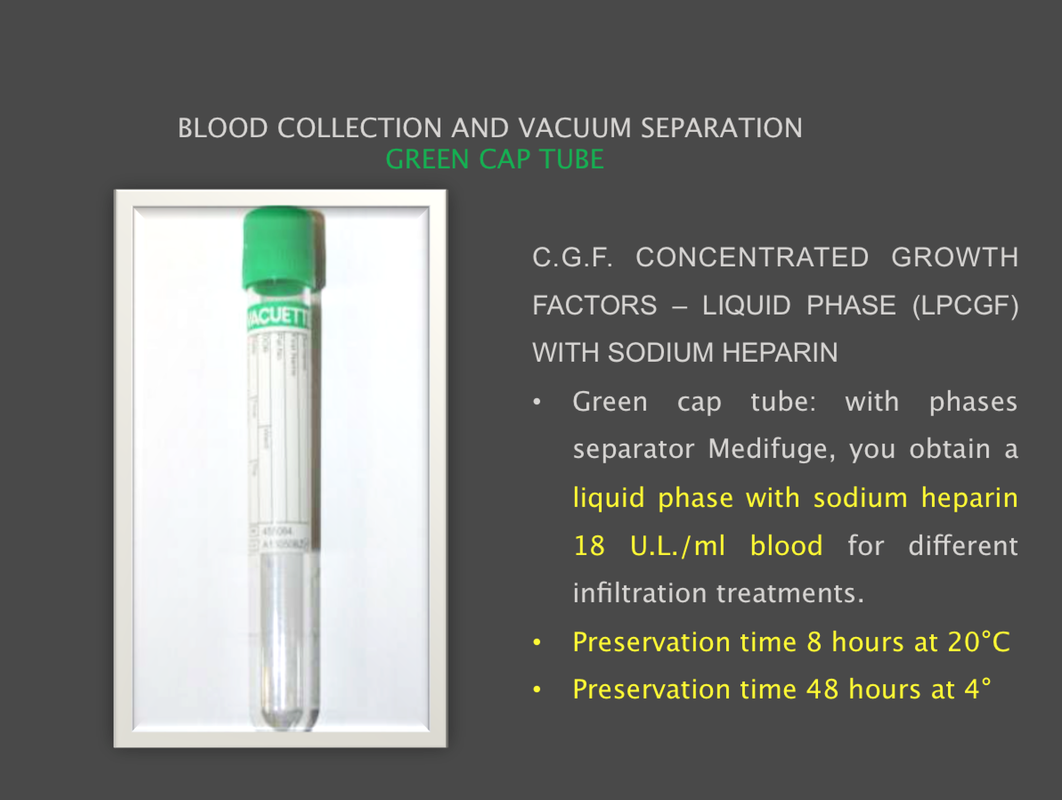
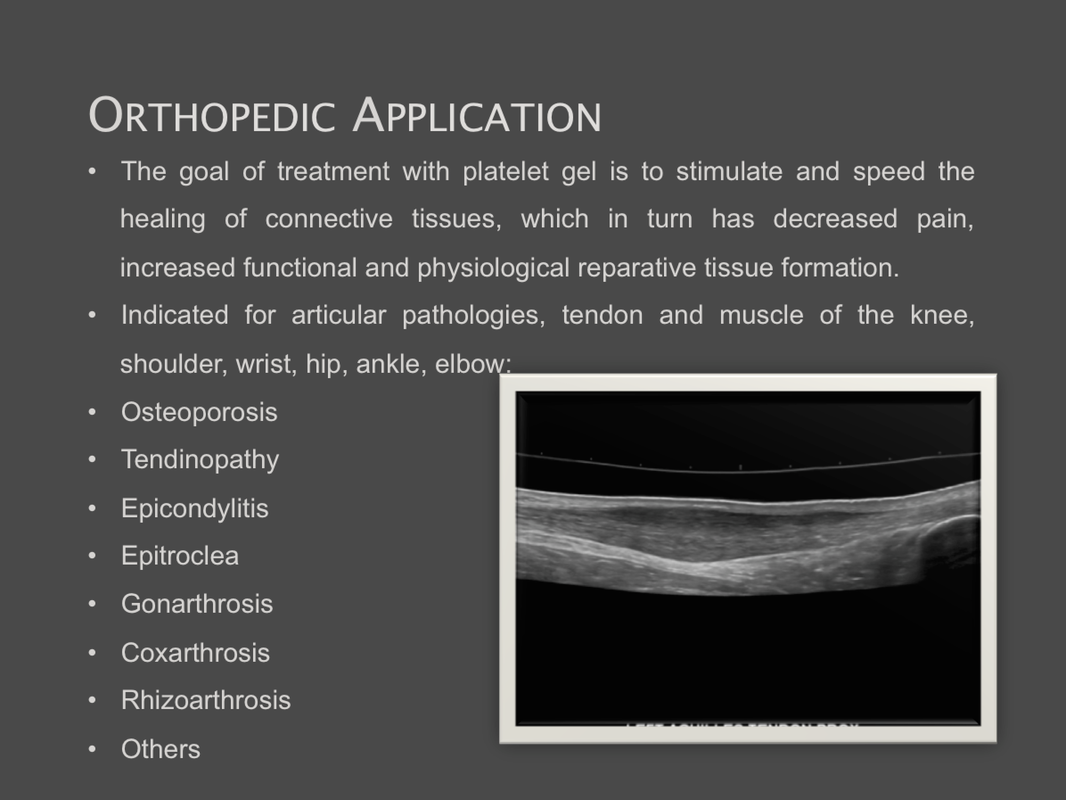
The Silfradent Medifuge MF200 is a dedicated medical plasma centrifuge. It is designed to prepare autologous concentrated growth factor (CGF) and CD 34 + cells using patient's own blood wich is the ideal autologous source without the addition of exogenous substances. CGF represents a new generation of platelet that able to hold inside a higher concentration of autologus. Growth factors are proteins which regulate the complex processes of wound healing and enhance the body's healing abilities without side-effects. The Medifuge prepares up to 8 test tubes of various levels of concentrate with active proteins in liquified or Fibrin form to create membranes, glue or other particulars for tissue regeneration and bone augmentation in a short period of 12 minutes. Medifuge MF200 Technical Specifications: Product Features:
MEDIFUGE & CGF KITPatent pending - Blood Phase Separator
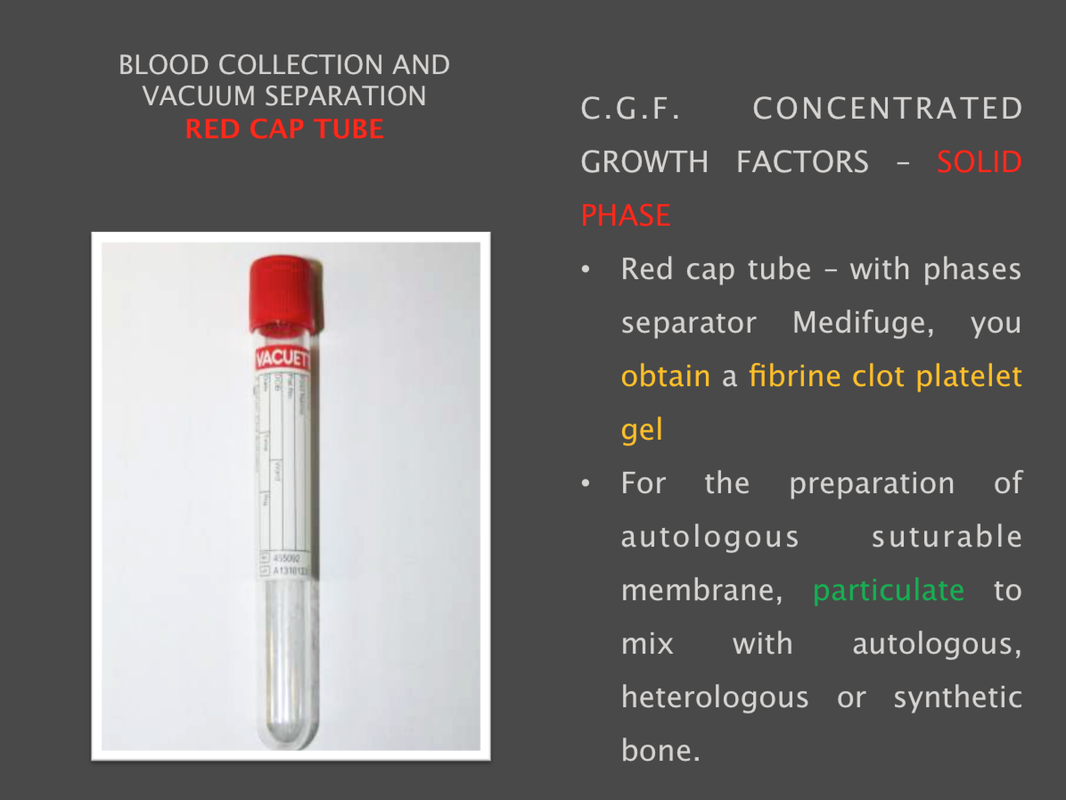
CGF KITThe patent kit is equipped with the neccessary for blood collection, specific dappens to prevent any contamination risks and the required instrument to produce the fibrin membrane and insertion of the clot in the site. Capacity: 8 units (test tubes) Processing Time12 minutes Power Source: 230V +/- 10% 50/60 Hz Noise Level: Don't exceed 57 dB(A) Dimensions of Unit: 230w x 320L x 240H mm Weight: 9.4 Kg Country of Manufacture: Italy |
Sifradent’s advance: •Controlled acceleration •Cycle Bleaching sterilizing= decontamination •Antistatic, anti-magnetic rotor •Self-ventilation to control temperature •Deceleration control •Cooling •Temperature control •Self-decontamination •Rotor with alternate and controlled speed with an acceleration always under RCF300 |
|
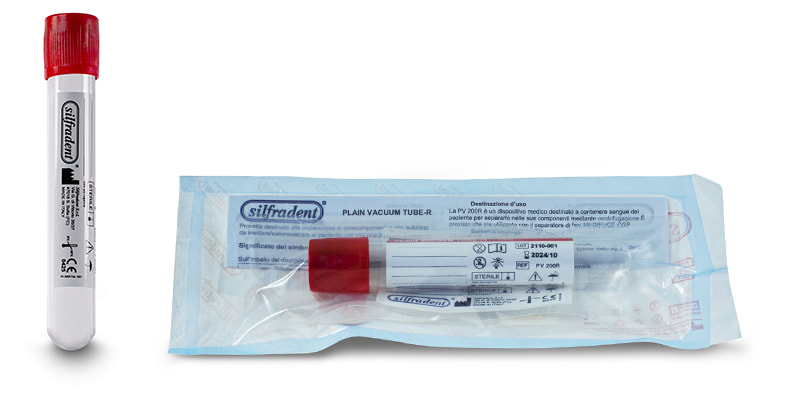
Test tube PV 200R (unitary code) 10 ml
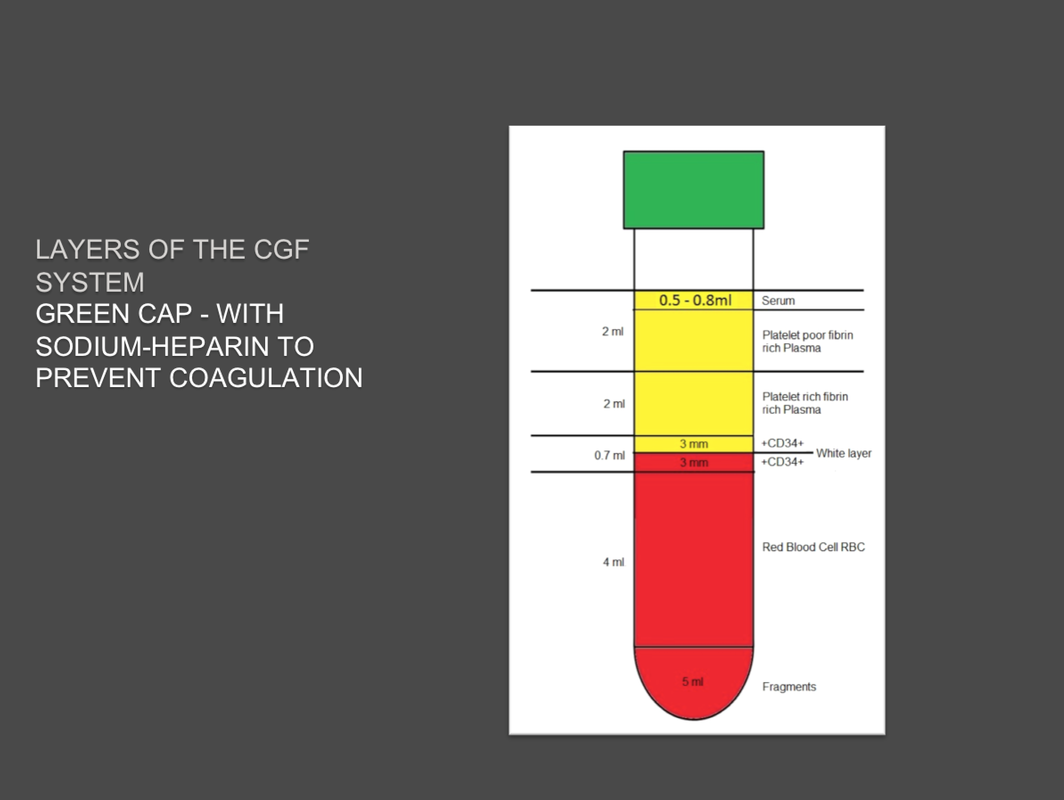
Glass plain test tube empty to produce CGF (Concentrated Growth Factors) solid Plasma. Pack of 50 test tubes: single unit packed in double sterile bag. |
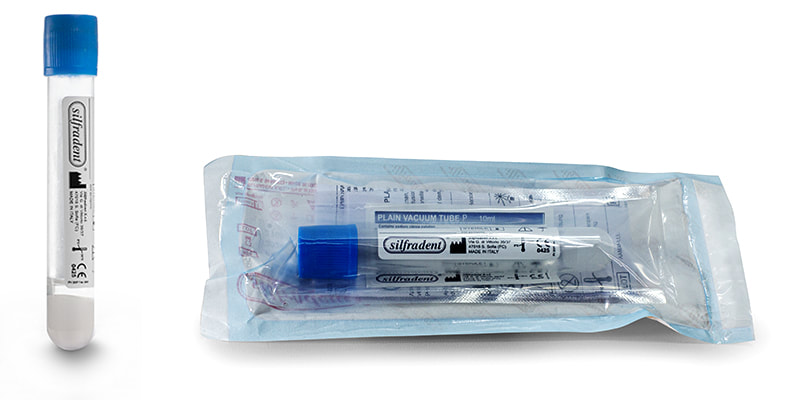
Test tube PV 200P (unitary code) 10 ml
Glass test tube with separator gel and sodium citrate anticoagulant: to produce liquid plasma PRP (Platelet Rich Plasma). Pack of 20 test tubes: single unit packed in double sterile bag. Clicca qui per modificare. |